Rofekoksib
 | |||
| (IUPAC) ime | |||
|---|---|---|---|
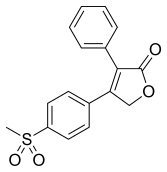
| 4-(4-metilsulfonilfenil)-3-fenil-5H-furan-2-on | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 162011-90-7 | ||
| ATC kod | M01AH02 | ||
| PubChem[1][2] | 5090 | ||
| DrugBank | APRD00151 | ||
| ChemSpider[3] | 4911 | ||
| UNII | 0QTW8Z7MCR  Y Y | ||
| KEGG[4] | D00568  Y Y | ||
| ChEBI | CHEBI:8887  Y Y | ||
| ChEMBL[5] | CHEMBL122  Y Y | ||
| Hemijski podaci | |||
| Formula | C17H14O4S | ||
| Mol. masa | 314.357 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Bioraspoloživost | 93% | ||
| Vezivanje za proteine plazme | 87% | ||
| Metabolizam | hepatički | ||
| Poluvreme eliminacije | 17 sati | ||
| Izlučivanje | bilijarno/renalno | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | povučen sa tržišta | ||
| Način primene | oralno | ||
Rofekoksib je nesteroidni antiinflamatorni lek (NSAID) koji je povučen sa tržišta iz bezbednostnih razloga. Njega je razvila kompanija Merk (Merck & Co.) za lečenje osteoartritisa, stanja akutnog bola i dismenoreja. Rofekoksib je FDA odobrila 20. maja 1999. On je bio u prodaji pod imenima Vioxx, Ceoxx, i Ceeoxx.
Rofekoksib je postao široko prihvaćen među lekarima koji su tretirali pacijente sa artritisom i drugim oboljenjima koja izazivaju hronični ili akutni bol. Svojevremeno je širom sveta ovaj lek je koristilo oko 80 miliona ljudi.[6]
Merk je 30. septembra 2004 povukao rofekoksib sa tržišta usled zabrinutosti zbog povećanog rizika od srčanog udara vezanog za dugotrajnu upotrebu visokih doza leka. Do povlačenja je došlo nakon obelodanjivanja da informacije o rizicima nisu bile objavljene više od pet godina. To je uzrokovalo između 88,000 i 140,000 slučajeva ozbiljnih oboljenja srca.[7] Rofekoksib je bio jedan od najšire korištenih lekova ikad povučenih sa tržišta. U toku godine pre povlačenja, Merkov prihod od prodaje Vajoksa je bio US$2.5 milijarde.[8]
Rofekoksib je bio dostupan na recept, u obliku tableta i kao oralna suspenzija. U obliku injekcija je prodavan za bolničku upotrebu.
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ „Merck Pulls Arthritis Drug Vioxx from Market”.
- ↑ Slate (14. 11. 2011.). „The Cover-Up Artist”. Slate. str. 1.
- ↑ Reuters (07. 12. 2006.). „Merck Sees Slightly Higher 2007 Earnings”. New York Times. str. A1.
Literatura
- Nicholas C. Price, Lewis Stevens (1999). Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins (Third izd.). USA: Oxford University Press. ISBN 019850229X.
- Eric J. Toone (2006). Advances in Enzymology and Related Areas of Molecular Biology, Protein Evolution (Volume 75 izd.). Wiley-Interscience. ISBN 0471205036.
- Branden C, Tooze J.. Introduction to Protein Structure. New York, NY: Garland Publishing. ISBN: 0-8153-2305-0.
- Irwin H. Segel. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems (Book 44 izd.). Wiley Classics Library. ISBN 0471303097.
- Robert A. Copeland (2013). Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists (2nd izd.). Wiley-Interscience. ISBN 111848813X.
- Gerhard Michal, Dietmar Schomburg (2012). Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology (2nd izd.). Wiley. ISBN 0470146842.
- FDA (2005). "Summary minutes for the February 16, 17 and 18, 2005, Joint meeting of the Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee." Published on the internet, March 2005. Link
- Fitzgerald GA, Coxibs and Cardiovascular Disease, N Engl J Med 2004;351(17): 1709–1711. PMID 15470192.
- Grassley CE (15 Oct 2004). Grassley questions Merck about communication with the FDA on Vioxx. Arhivirano 2004-10-29 na Wayback Machine-u Press Release.
- Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M (2004). Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet (published online; see also Merck response below)
- Karha J and Topol EJ. The sad story of Vioxx, and what we should learn from it Arhivirano 2008-04-06 na Wayback Machine-u Cleve Clin J Med 2004; 71(12):933-939. PMID 15641522
- Michaels, D. (June 2005) DOUBT Is Their Product, Scientific American, 292 (6).
- Merck & Co., (5 Nov 2004). Response to Article by Juni et al. Published in The Lancet on Nov. 5. Arhivirano 2004-12-21 na Wayback Machine-u Press Release.
- Merck & Co (30 Sep 2004) Merck Announces Voluntary Worldwide Withdrawal of VIOXX. Press release [1].
- D. M. Mukherjee, S. E. Nissen, and E. J. Topol, “Risk of Cardiovascular Events Associated with Selective COX-2 Inhibitors,” Journal of the American Medical Association 186 (2001): 954–959.
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005;352(11):1081-91. PMID 15713945
- Okie, S (2005) "Raising the safety bar--the FDA's coxib meeting." N Engl J Med. 2005 Mar 31;352(13):1283-5. PMID 15800221.
- Leleti Rajender Reddy, Corey EJ. Facile air oxidation of the conjugate base of rofecoxib (Vioxx), a possible contributor to chronic human toxicity Tetrahedron Lett 2005, 46: 927. [2]
- Swan SK et al., Effect of Cyclooxygenase-2 Inhibition on Renal Function in Elderly Persons Receiving a Low-Salt Diet. Annals of Int Med 2000; 133:1–9
- Targum, SL. (1 Feb. 2001) Review of cardiovascular safety database. FDA memorandum. [3]
- Wolfe, MM et al., Gastrointestinal Toxicity of Nonsteroidal Anti-anflamattory Drugs, New England Journal of Medicine. 1999; 340; 1888-98.
Spoljašnje veze
- Reakcija nakon povlačenja leka
- Sudski postupak
- p
- r
- u
(profeni)
(fenaminske kiseline)





