Pindobind
| Pindobind | |||
|---|---|---|---|
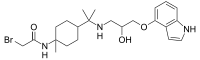 | |||
| IUPAC ime |
| ||
| Identifikacija | |||
| CAS registarski broj | 106469-52-7  Y Y | ||
| PubChem[1][2] | 4827 | ||
| ChemSpider[3] | 4661 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C23H34BrN3O3 | ||
| Molarna masa | 480.44 g mol−1 | ||
|
| |||
| Infobox references | |||
Pindobind je hemikalija koji je patentirao IBM.[4] On je eksperimentalno identifikovan kao depresant centralnog nervnog sistema.[5] On kod životinja uzrokuje smanjenje ofanzivnih akcija, kao što je jurinje.[5]
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ PubChem 4827
- ↑ 5,0 5,1 Bell, R; Hobson, H (1993). „Effects of pindobind 5-hydroxytryptamine1A (5-HT1A), a novel and potent 5-HT1A antagonist, on social and agonistic behaviour in male albino mice”. Pharmacology, Biochemistry, and Behavior 46 (1): 67–72. DOI:10.1016/0091-3057(93)90318-N. PMID 8255924.










