Syringin
 | |
| Names | |
|---|---|
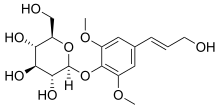
| IUPAC name 4-[(1E)-3-Hydroxyprop-1-en-1-yl]-2,6-dimethoxyphenyl β-D-glucopyranoside | |
| Systematic IUPAC name (2R,3S,4S,5R,6S)-2-(Hydroxymethyl)-6-{4-[(1E)-3-hydroxyprop-1-en-1-yl]-2,6-dimethoxyphenoxy}oxane-3,4,5-triol | |
| Other names Eleutheroside B; Ilexanthin A; Ligustrin; Lilacin; Magnolenin; Methoxyconiferine; Sinapyl alcohol 4-O-glucoside; Siringin; Syringoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.120.487 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C17H24O9 |
| Molar mass | 372.370 g·mol−1 |
| Appearance | White crystalline solid |
| Melting point | 192 °C (378 °F; 465 K)[1] |
Solubility in water | Slightly soluble[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Syringin is a natural chemical compound first isolated from the bark of lilac (Syringa vulgaris) by Meillet in 1841.[2][1] It has since been found to be distributed widely throughout many types of plants. It is also called eleutheroside B, and is found in Eleutherococcus senticosus (Siberian ginseng). It is also found in dandelion coffee. Syringin may potentially have antidiabetic effects.[3]
Chemically, it is the glucoside of sinapyl alcohol.
References
- ^ a b c Merck Index, 11th Edition, 8997
- ^ Park, Hee-Juhn; Jung, Won-Tae; Basnet, Purusotam; Kadota, Shigetoshi; Namba, Tsuneo (1996). "Syringin 4-O-β-Glucoside, a New Phenylpropanoid Glycoside, and Costunolide, a Nitric Oxide Synthase Inhibitor, from the Stem Bark of Magnolia sieboldii". Journal of Natural Products. 59 (12): 1128–1130. doi:10.1021/np960452i. PMID 8988596.
- ^ Sundaram Chinna Krishnan, Shanmuga; Pillai Subramanian, Iyyam; Pillai Subramanian, Sorimuthu (2014). "Isolation, characterization of syringin, phenylpropanoid glycoside from Musa paradisiaca tepal extract and evaluation of its antidiabetic effect in streptozotocin-induced diabetic rats". Biomedicine & Preventive Nutrition. 4 (2): 105–111. doi:10.1016/j.bionut.2013.12.009.
External links
 Media related to Syringin at Wikimedia Commons
Media related to Syringin at Wikimedia Commons
- v
- t
- e











