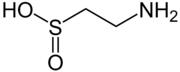
Hypotaurine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Aminoethane-1-sulfinic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.155.825 |
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C2H7NO2S |
| Molar mass | 109.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Hypotaurine is a sulfinic acid that is an intermediate in the biosynthesis of taurine. Like taurine, it also acts as an endogenous neurotransmitter via action on the glycine receptors.[1] It is an osmolyte with antioxidant properties.[2]
Hypotaurine is derived from cysteine (and homocysteine). In mammals, the biosynthesis of hypotaurine from cysteine occurs in the pancreas. In the cysteine sulfinic acid pathway, cysteine is first oxidized to its sulfinic acid, catalyzed by the enzyme cysteine dioxygenase. Cysteine sulfinic acid, in turn, is decarboxylated by sulfinoalanine decarboxylase to form hypotaurine. Hypotaurine is enzymatically oxidized to yield taurine by hypotaurine dehydrogenase.[3]

References
- ^ Kalir, Asher; Kalir, Henry H. "Biological activity of sulfinic acid derivatives" in Chemistry of Sulphinic Acids, Esters Their Derivatives Edited by Patai, Saul. Wiley, New York, 1990, pp. 665.
- ^ Paul H. Yancey (2005). "Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses". Journal of Experimental Biology. 208 (15): 2819–2830. doi:10.1242/jeb.01730. PMID 16043587.
- ^ Sumizu K (1962). "Oxidation of hypotaurine in rat liver". Biochim. Biophys. Acta. 63: 210–212. doi:10.1016/0006-3002(62)90357-8. PMID 13979247.
- v
- t
- e
| Major excitatory / inhibitory systems |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biogenic amines |
| ||||||||
| Neuropeptides |
| ||||||||
| Endocannabinoids | |||
|---|---|---|---|
| Neurosteroids |
| ||
| Nucleosides |
|
|---|
| Cholinergic system |
|---|
| Gasotransmitters |
|
|---|
| Candidates |
|
|---|
 | This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










